Use this link to read: White Paper - OA as a Serious Disease
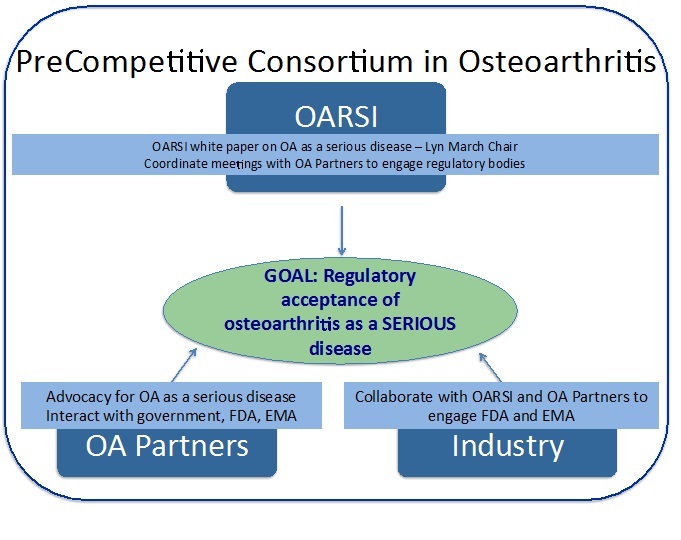
Pre-Competitive Consortium for Osteoarthritis
Osteoarthritis Research Society International
The pathway for developing structure modifying drugs for osteoarthritis (OA) has been complicated by the fact that OA is a heterogeneous disease process and by the inability to predict which patients will progressively lose function and develop more joint damage. This has led to an effort through the Osteoarthritis Initiative of the National Institute of Health (OAI-NIH) to define the natural history of a cohort of representative OA patients. Additionally, in 2010 the Osteoarthritis Research Society International (OARSI), in response to a Food and Drug Administration (FDA) Federal Register notice requesting additional information on issues related to clinical development programs for drugs and medical devices for the treatment and prevention of OA, provided feedback to the FDA on relevant questions related to OA assessment and clinical trial design. Subsequently, in 2011, OARSI in collaboration with the Foundation for the NIH (FNIH) initiated a 2.5-year study to further explore imaging and biomarkers as predictors of disease progression and clinical outcomes.
The mission of the Pre Competitive Consortium for Osteoarthritis (PCCOA) is to advance development of structure modifying therapies for the treatment of patients with OA. The proposed strategy is to enhance collaboration among stakeholders and promote the sharing of knowledge of barriers and opportunities for OA drug discovery and development. It is anticipated that a new synthesis of the expertise of academic researchers, clinical investigators, and representatives from pharmaceutical and biotechnology R&D segments can effectively inform the drug approval process about ways to remove the current barriers to new treatments for OA. The current US Food and Drug (FDA) approval process for structure modifying drugs for OA requires concomitant improvement in a qualified and validated imaging or biochemical marker and improvement in the signs and symptoms of the disease. This creates formidable challenges for the development of structure modifying drugs for OA. The aim of the PCCOA is to design a pathway to establish the importance of OA as a serious condition for which there are currently no satisfactory therapies according to the FDA definition:
The PCCOA will oversee the development of a white paper supporting the argument that OA is a serious condition. This will require an extensive review of the epidemiology of OA, impact on quality of life, associated symptoms and functional disability, and association with increased risk of comorbidity and mortality. An additional systematic review of clinically relevant outcomes in OA will be undertaken to establish the argument that biomarker (biochemical and/or imaging) intermediate endpoints can serve as surrogates of structural change endpoints and that the measured changes are meaningful as they relate to the clinical outcomes of pain and stiffness, need for assistive devices or need for joint replacement. Lastly, but most importantly, the PCCOA will collaborate with patients to gain their perspectives which will further inform the strategic messaging to be delivered to governmental agencies within the US and Europe.
For more information, contact Valorie Thompson ( vthompson@mac.com)
Thank you to the industry supporters of this initiative:
EMD Serono
Fidia Pharmaceuticals
Flexion
Nordic Biosciences
Spinifex
| PCCOA Executive Committee | PCCOA Writing Group (Co-Chairs and Proposed Members) | |
|---|---|---|
| Francis Berenbaum, MD, PhD Head, Department of Rheumatology Faculty of Medicine Pierre & Marie Curie Paris VI Saint-Antoine Hospital Paris, France |
Lyn March, MB BS, MSc, PhD (Co-Chair) Professor of Medicine and Public Health Senior Staff Specialist in Rheumatology and Clinical Epidemiology Institute of Bone and Joint Research Sydney Medical School University of Sydney Sydney, NSW, Australia |
|
| Philip G. Conaghan, MB BS, PhD Professor of Musculoskeletal Medicine University of Leeds Consultant Rheumatologist Leeds Teaching Hospitals NHS Trust & Leeds Primary Care Trust Deputy Director, Leeds NIHR Biomedical Research Unit Leeds, UK |
Gillian Hawker, MD (Co-Chair) Senior Scientist, Women’s College Research Institute Chair, Department of Medicine Sir John and Lady Eaton Professor, Department of Medicine Professor, Institute of Health Policy, Management and Evaluation University of Toronto Toronto, Canada |
|
| Gillian Hawker, MD Senior Scientist, Women’s College Research Institute Chair, Department of Medicine Sir John and Lady Eaton Professor, Department of Medicine Professor, Institute of Health Policy, Management and Evaluation University of Toronto Toronto, Canada |
Nigel Arden, MBBBS, FRCP, MSc, MD Professor in Rheumatic Diseases and Consultant Rheumatologist University of Southampton Southampton University Hospitals NHS Trust Reader in Musculoskeletal Sciences and Consultant Rheumatologist Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences Botnar Research Centre University of Oxford United Kingdom |
|
| Marc C. Hochberg, MD, MPH Professor of Medicine, Epidemiology and Public Health Head, Division of Rheumatology & Clinical Immunology Vice Chair, Department of Medicine University of Maryland School of Medicine Baltimore, Maryland, USA |
Marita Cross, BSc, MPH, PhD |
|
|
Virginia Byers Kraus, MD, PhD |
David Felson, MD NIHR Manchester Musculoskeletal Biomedical Research Unit University of Manchester United Kingdom Director, Clinical Epidemiology Professor of Medicine and Public Health Boston University School of Medicine Boston, Massachusetts USA |
|
| Lee S. Simon, MD Former Division Director FDA Analgesic, Anti-inflammatory, Ophthalmologic Drug Products Division (CDER) Principal, SDG LLC Cambridge, Massachusetts, USA |
Francis Guillemin, MD, PhD Professor of Epidemiology and Public Health School of Public Health, Faculty of Medicine University of Nancy France |
|
| Catherine Hill, MBBS, MSc, FRACP Consultant Rheumatologist The Queen Elizabeth Hospital, Adelaide Woodville, South Australia Australia |
||
| Graeme Jones, MBBS, FRACP, FAFPHM, MMedSC, MD Professor of Rheumatology and Epidemiology Head, Musculoskeletal Unit, Menzies Research Institute University of Tasmania Head, Department of Rheumatology, Royal Hobart Hospital Hobart, TAS, Australia |
||
|
Tore Kvien, MD, PhD |
||
| Michael Nevitt, PhD, MPH Professor, University of California San Francisco School of Medicine Department of Epidemiology and Biostatistics, San Francisco California, USA |